Pathophysiology of Vitreomacular Traction
In symptomatic VMA, the continuous tangential and antero posterior stretching on the macula can lead to VMT,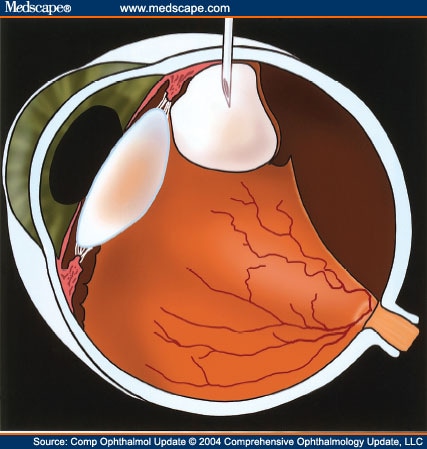 w
w
hich, if allowed to progress, can lead to sight-threatening complications, such as the formation of macular edema and macular hole. VMT can also lead to microscopic damage of the retinal surface. When this focal area of damage or irritation occurs in the macular region, the retina initiates a healing response, leading to a thin layer of scar tissue known as macular pucker or epiretinal membrane. A review of the electron microscopic features of the preretinal membrane tissue removed from eyes with VMT showed a high prevalence of fibroglial tissue, including thickened internal limiting membrane, astrocytes and myofibrocytes. w
w
Abnormalities at the vitreomacular interface, such as retinoschisis progressing to macular hole, have also been reported in the setting of high myopia. This may relate to the higher axial length; an axis length of over 31 mm was reported a risk factor. Other risk factors included macular chorioretinal atrophy and vitreoretinal interface disorders, including macular pucker, posterior vitreoschisis and VMT. The clinical presentation of abnormalities at the vitreoretinal interface may differ depending on the size and strength of the remaining vitreomacular attachments, and on the structural integrity of the macula. For instance, macular hole associated with VMT is found to have a narrower diameter VMA compared with macular edema. It seems that the narrower the VMA, the greater the force it exerts upon the macula, which can lead to macular hole.
Macular holes progress from stage 0 to 4, where stage 0 is only noticeable with optical coherence tomography (OCT; see also section below) and stage 4 is a fully developed hole with complete posterior vitreous detachment. VMT has been associated with the development of macular holes in the literature. An anterior–posterior tractional force contributes to a prehole intraretinal split, which is thought to precede the formation of an idiopathic full-thickness macular hole.
Anatomy & Physiology of the Vitreous & the Vitreoretinal Interface
The vitreous gel is a transparent extracellular matrix that fills the cavity behind the lens of the eye. It occupies an average volume of 4.4 ml in adulthood. It is surrounded by and attached to the retina and lens of the eye.[1] It is a virtually acellular, highly hydrated extracellular gel matrix, composed of approximately 99% water.[15] The transparent nature of the vitreous makes it challenging to study its structure and anatomy.
The gel structure is maintained by a dilute network of thin, unbranched collagen fibrils that are mixed (heterotopic) in composition, comprising collagen types II, V/XI and IX in a molar ratio of 75:10:15, respectively. Collagen type V/XI forms the core of the fibrils, with type II surrounding the core and type IX on the outside of the fibril. The spaces between these collagen fibrils are mostly filled by glycosaminoglycans (GAGs), mainly hyaluronan. Opticin has been found to coat the collagen fibers and, by binding to different GAGs, may play a role in maintaining the gel structure of the vitreous and also in adhesion at the vitreoretinal interface. Chondroitin sulfate, another GAG linked to type IX collagen, was found to play a role in bridging adjacent collagen fibrils and at the same time spacing them apart to minimize light scattering and maintain vitreous transparency. The vitreoretinal interface is an adhesive sheet that facilitates the connection of the posterior vitreous cortex of the vitreous body to the internal limiting membrane of the retina.The vitreoretinal interface consists of matrix proteins including laminin, fibronectin and collagen IV, and it is thought that these may act as an extracellular matrix 'glue'.
It is not completely understood how the vitreous and retina interact. The vitreous is known to be most firmly attached at places where the internal limiting membrane is thinnest, that is, the vitreous base, optic disc and macula, and over retinal blood vessels.At the base area, the vitreous and the retina are connected by vitreous collagen fibers passing though the internal limiting membrane and intertwining with retinal collagen. The collagen fibrils of the posterior vitreous cortex are indirectly attached to the internal limiting membrane, and hence to the retina via laminin and fibronectin. The attachment between the vitreous and the macula is called the vitreomacular interface.
Treatment Paradigm for Vitreomacular Interface Diseases
Patients with symptomatic VMA including macular hole have a poor prognosis if left untreated. Most untreated eyes undergo a further decrease in vision and in some cases progressive complications. In patients with symptomatic VMA who underwent vitrectomy, greater improvement in visual acuity was observed in eyes with better preoperative visual acuity and shorter duration of symptoms. Thus, the data suggest that earlier treatment of symptomatic VMA and related diseases may help in achieving better visual outcome. Nevertheless, the invasiveness, patient inconvenience and complications commonly associated with vitrectomy, as mentioned previously, often limit its use to patients with advanced disease. Therefore, patients often remain untreated until the condition progressively worsens to a point that warrants surgery.
The goal of therapy for symptomatic VMA and related conditions is to relieve tractional effects on the macula, thereby resolving the underlying condition with subsequent functional improvement. The only treatment option available currently to achieve this goal is surgery (vitrectomy). A minimally invasive, less traumatic and well-tolerated pharmacological treatment option for this progressive and potentially sight-threatening condition would represent a significant advance in care. Currently, there is no pharmacological treatment available for symptomatic VMA and related conditions.
No comments:
Post a Comment